BASES
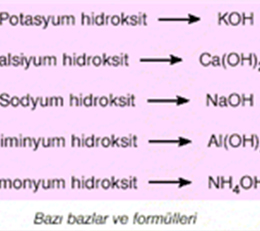 Alkali is a substance that can provide OH ions and electron pairs to the environment when ionized in water. Alkali is a dangerous substance like acid. Substances that increase the concentration of hydroxide (OH-) ions when ionized in water are called alkalis.
Alkali is a substance that can provide OH ions and electron pairs to the environment when ionized in water. Alkali is a dangerous substance like acid. Substances that increase the concentration of hydroxide (OH-) ions when ionized in water are called alkalis.The dissociation of some alkalis into ions in aqueous solutions is as described above. However, although ammonia (NH3) does not contain hydroxide ions, it shows basic properties. Because this will lead to an increase in the concentration of OH ions in its aqueous solution. NH3 + H2O → NH4 + + OH−
The sole is slippery in the hands. A strong base is corrosive and irritating. Alkaline is bitter, but some types of alkali are toxic. Therefore, it should not be tried.
Like acid, alkali can be distinguished by litmus paper. (Litmus material is obtained from lichen.) Alkali turns red litmus paper blue.
In addition, bases can be distinguished by phenolphthalein solution. When the phenolphthalein solution becomes alkaline, the alkali turns pink. Phenolphthalein will not change the color of the acid when placed in acid. When alkaline (like acid) dissociates into ions in water, it conducts electric current.
NaOH and KOH are strong bases. Strong alkalis can corrode metals and irritate tissues Ammonia can damage eyes, nose and respiratory system.